ABOUT THE FR104/VEL-101 CLINICAL PROGRAM
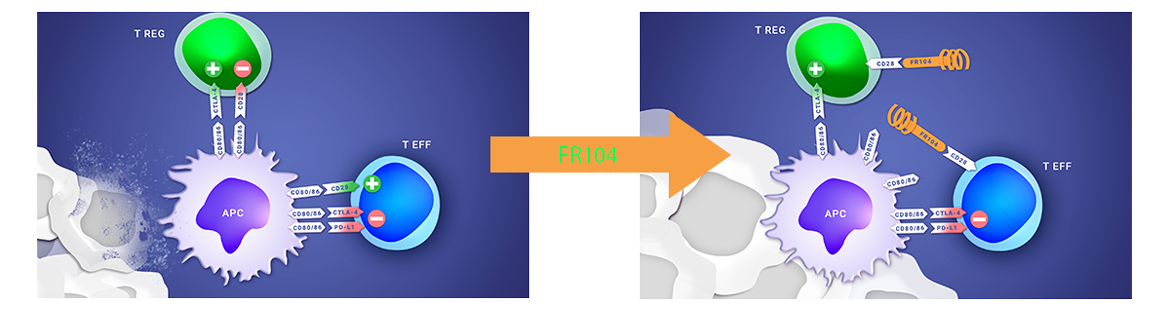
FR104’s Phase 1 clinical study has shown initial signals of efficacy, a good safety profile and the recommended dose for a Phase 2, further supporting the continued clinical development of this asset in autoimmune diseases or in transplantation.
Poirier et al. ; First-in-Human Study in Healthy Subjects with FR104, a Pegylated Monoclonal Antibody Fragment Antagonist of CD28 ; Journal of Immunology; 2016
FR104/VEL-101 is under a global license agreement (April 2021) with Veloxis Pharmaceuticals Inc., a leading transplantation company, to develop, manufacture and commercialize FR104 for all transplant indications. Based on preclinical and Phase 1 successful studies, Veloxis plans to advance FR104/VEL-101 development to provide a new therapeutic option for prophylaxis of organ rejection in patients receiving a solid organ transplant. An international Phase 2 clinical trial in kidney transplantation is under preparation by Veloxis.
A Phase 1/2 trial (FIRsT study) is evaluating first administration of FR104, in patients undergoing renal transplant. This study is being conducted as part of a collaboration agreement between OSE Immunotherapeutics and the University Hospital of Nantes, the trial’s sponsor. Patient enrollment is completed and a longer-term follow-up assessment is planned to be performed one year after transplantation.
The purpose of the FIRsT study is to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of FR104 in renal transplant patients. (ClinicalTrials.gov : NCT04837092)